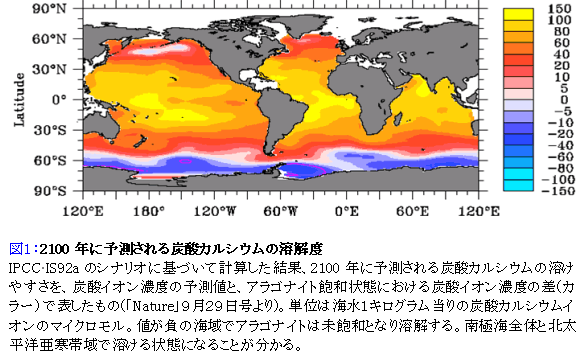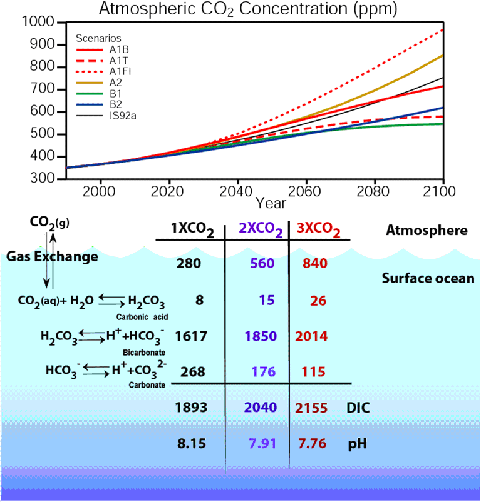
Summary of surface ocean carbon chemistry with rising atmospheric
CO2 levels
Air-sea gas exchange is a physico-chemical process,
primarily controlled by the air-sea difference in gas concentrations
and the exchange coefficient, which determines how quickly a
molecule of gas can move across the ocean-atmosphere boundary.
It takes about one year to equilibrate CO2 in the surface ocean with atmospheric CO2, so it is not unusual to observe
large air-sea differences in CO2 concentrations. Most of the differences are caused
by variability in the oceans due to biology and ocean circulation.
The oceans contain a very large reservoir of carbon that can
be exchanged with the atmosphere because the CO2 reacts with water to form carbonic acid and its dissociation
products. As atmospheric CO2 increases in the future, the interaction with the surface
ocean will change the chemistry of the seawater. The total dissolved
inorganic carbon (DIC) content of the waters will increase.
Because CO2 is an acid
gas, the carbonate ion (CO32-) concentration
and pH of the surface waters will decrease. The physiological
effects of these changes on ocean biology are not fully understood,
but studies have suggested that these changes may decrease calcification
rates in coccoliths, corals, and coralline algae.
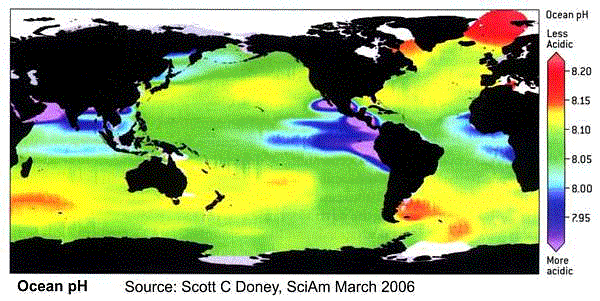
〔NOAAのPMEL CO2 Programから〕
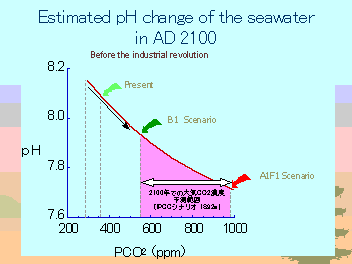
現在の海水は pH 8.15 程度であるが、大気中二酸化炭素濃度が2倍になれば pH 7.91 に、3倍になれば pH
7.76 になると予想される。 |