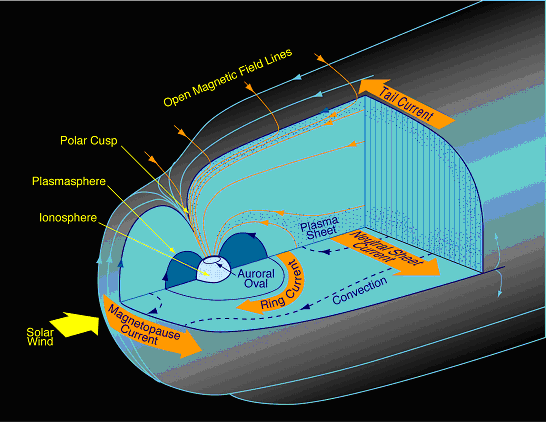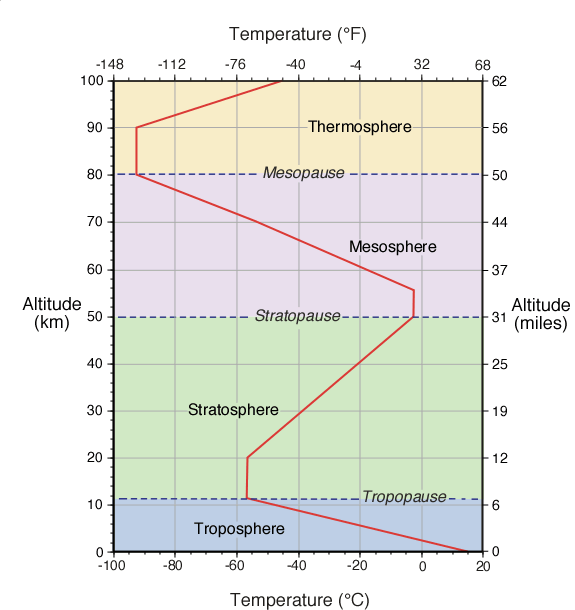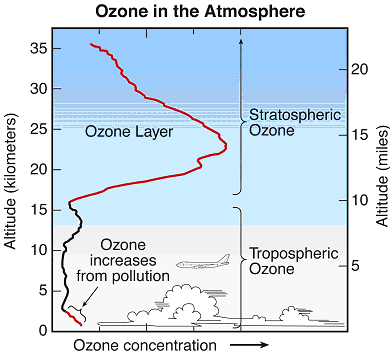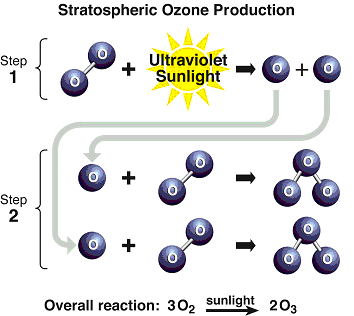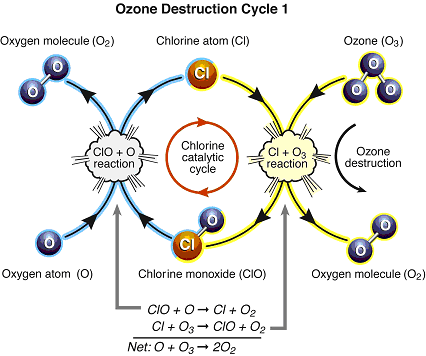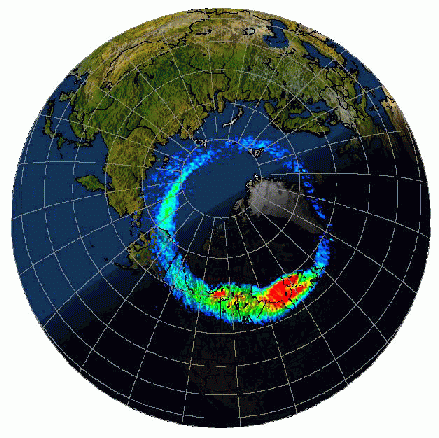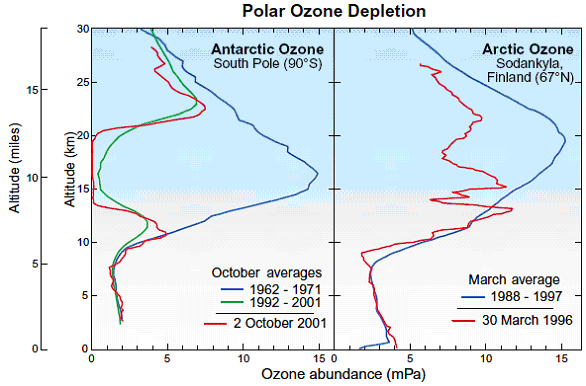
Figure Q11-2. Arctic and Antarctic ozone distribution. The stratospheric ozone layer resides between about
10 and 50 kilometers (6 to 31 miles) above Earth’s surface over the globe. Long-term observations
of the ozone layer from small balloons allow the winter Antarctic
and Arctic regions to be compared. In the Antarctic at the South
Pole, halogen gases have destroyed ozone in the ozone layer beginning
in the 1980s. Before that period, the ozone layer was clearly
present as shown here using average ozone values from balloon
observations made between 1962 and 1971. In more recent years,
as shown here for 2 October 2001, ozone is destroyed completely
between 14 and 20 kilometers (8 to 12 miles) in the Antarctic in spring. Average October values
in the ozone layer now are reduced by 90% from pre-1980 values.
The Arctic ozone layer is still present in spring as shown by
the average March profile obtained over Finland between 1988
and 1997. However, March Arctic ozone values in some years are
often below normal average values as shown here for 30 March
1996. In such years, winter minimum temperatures are generally
below PSC formation temperatures for long periods. (Ozone abundances are shown here with the unit “milli-Pascals”
(mPa), which is a measure of absolute pressure (100 million mPa
= atmospheric sea-level pressure).)
〔NOAA Aeronomy Laboratoryの『International
Ozone-Layer Assessments』の『WMO/UNEP
Scientific Assessment of Ozone Depletion: 2002』の『Twenty
Questions and Answers About the Ozone Layer』の『III STRATOSPHERIC
OZONE DEPLETION』の中の『Q11:
How severe is the depletion of the Antarctic ozone layer?』から〕
南極と北極上空のオゾン層が破壊されていることを示す観測データ。フロンが犯人であるが、両極とくに南極で破壊が著しいのは極域成層圏雲が重要な役割をしていると考えられている。 |