本文で議論される芳香族カルボン酸の構造
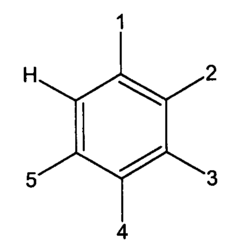
** = represents double bond.
*** R1 represents CH2C[OH][COOH]CH[OH]COOH where [ ] represents branched group.
『Abstract
Concentrations of organic acids in prebiotic soils were presumably
low, given limitations in abiotic synthesis and the limited lifetimes
of organic molecules before the ultraviolet shield developed on
early Earth. Prokaryotes, the first land-colonizing organisms,
commonly secrete aliphatic carboxylic acids, and, less extensively,
secrete aromatic compounds as siderophores and antibiotic. In
contrast, secretion of aromatic acids is considerable for fungi,
lichens, and vascular plants. Aromatic acids are also produced
by degradation oh high-molecular-weight compounds from lignin
and tannin, both abundant in vascular plants. The proportion of
aromatic carboxylic acids in soil solutions therefore probably
increased with the evolution of higher order organisms. As biomass
of organisms increased over geological time, concentrations of
organic acids in soil solutions and, in turn, the extent of ligand-promoted
dissolution of minerals probably increased.
To elucidate the contribution of ligands during weathering on
early Earth, Columbia River basalt was dissolved under oxic and
anoxic conditions in the presence (0.001 or 0.01 M) and absence
of several organic ligands in batch experiments at pH 6. Release
of all elements including Si was enhanced considerably in the
presence of organic ligands. Citrate (tridentate) and gallate
(tetradentate) increased element release to the greatest extent
among the aliphatic and aromatic ligands, respectively. The extent
of element mobilization observed for the aliphatic ligands
decreased in the order: citrate>oxalate≒malonate, and for
the aromatic ligands: gallate>salicylate≒phthalate.
The effects of the ligands generally followed trends in cation-ligand
stability constants, but aromatic ligands were less effective
in element mobilization than aliphatic ligands. One exception
was gallate, an aromatic ligand, which significantly enhanced
Cu release. Ligand-promoted mobilization of Cu may therefore have
increased over geologic time with the increase in the proportion
of aromatic ligands.
In the presence of organic ligands, Fe was mobilized from basalt
considerably more than Al even under oxic conditions. Complexation
of Fe with organic ligands may have mobilized Fe in Precambrian
paleosols where little Al mobility is observed. Extent of P and
Y release was minor in ligand-free experiments and considerable
with ligands regardless of PO2.
Release of Cu was considerable under oxic conditions, especially
with ligands, and minor under anoxic conditions. Mobility patterns
of P and Y could thus possibly serve as “organomarkers” (indicative
of prevalence of organic ligands in soil solutions) and mobility
patterns of Cu could possibly serve as “oxymarkers” (indicative
of the presence of molecular oxygen), respectively, in ancient
soils.』
Introduction
Evolution of moieties of organic acids
Abiotic synthesis
General overview of biological evolution
Secretion of LMWOA by Prokaryotes
Secretion of LMWOA by fungi and lichens
LMWOA in plant root exudates
LMWOA as degradation products of HMW compounds
Summary of the evolution of organic acid moieties
Statement of objectives
Materials and methods
Organic ligands
Starting materials
Batch dissolution experiments
Results and discussion
Solution chemistry
Rate of dissolution
Ligand trends
Mobility of Si
Order of element release
Zr mobility
Implications of Fe and Al mobility patterns
Interpretations of P, Y and Cu mobility patterns
Cu, P, and Y in paleosols
Cu mobility over geologic time
Conclusions
Acknowledgments
Appendix 1
Appendix 2
References
|
|
|
|
|
| Acetic | 酢酸 | CH3COOH | 2 |
| Aconitic | アコニット酸 | HOOCC=[CHCOOH]CH2COOH | 6 |
| Butyric | 酪酸 | CH3(CH2)2COOH | 4 |
| Citric | クエン酸 | HOC[CH2COOH]2COOH | 6 |
| Formic | ギ酸 | HCOOH | 1 |
| Fumaric | フマル酸 | HOOCCH=CHCOOH | 4 |
| Glutaric | グルタル酸 | HOOC(CH2)3COOH | 5 |
| Glycolic | グリコール酸 | HOCH2COOH | 2 |
| Glyoxylic | グリオキシル酸 | OCHCOOH | 2 |
| Isocitric | イソクエン酸 | HOOCCH2CH[COOH]CH[COOH]OH | 6 |
| 2-ketogluconic | 2-ケトグルコン酸 | HOOCC[O](CH[OH])3CH2OH | 6 |
| α-ketoglutaric | α-ケトグルタル酸 | HOOC(CH2)2C[O]COOH | 5 |
| Lactic | 乳酸 | CH3CH[OH]COOH | 3 |
| Malic | リンゴ酸 | HOOCCH2CH[OH]COOH | 4 |
| Malonic | マロン酸 | HOOCCH2COOH | 3 |
| Oxalic | シュウ酸 | HOOCCOOH | 2 |
| Oxaloacetic | オキサロ酢酸 | HOOCC[O]CH2COOH | 4 |
| Propionic | プロピオン酸 | CH3CH2COOH | 3 |
| Pyruvic | ピルビン酸 | CH3C[O]COOH | 3 |
| Succinic | こはく酸 | HOOC(CH)2COOH | 4 |
| Tartaric | 酒石酸 | HOOC(CH[OH])2COOH | 4 |
| Valeric | 吉草酸 | CH3(CH2)3COOH | 5 |
| * [ ] represents branched group and = represents double bond. | |||
 |
||||||
|
|
|
|
||||
| 1 | 2 | 3 | 4 | 5 | ||
| Benzoic | 安息香酸 | COOH | H | H | H | H |
| Caffeic | コーヒ酸(カフェー酸) | CH=CHCOOH | H | OH | OH | H |
| Cinnamic | 肉桂酸(桂皮酸) | CH=CHCOOH | H | H | H | H |
| p-Coumaric | p-クマル酸 | CH=CHCOOH | H | H | OH | H |
| Ferulic | フェルラ酸 | CH=CHCOOH | H | OCH3 | OH | H |
| Gallic | 没食子酸 | COOH | H | OH | OH | OH |
| p-Hydrobenzoic | p-ヒドロ安息香酸 | COOH | H | H | OH | H |
| Phthalic | フタル酸 | COOH | COOH | H | H | H |
| Piscidic | R1*** | H | H | OH | H | |
| Salicylic | サリチル酸 | COOH | OH | H | H | H |
| Syringic | COOH | H | OCH3 | OH | OCH3 | |
| Terephthalic | テレフタル酸 | COOH | H | H | COOH | H |
| Vanillic | バニリン酸 | COOH | H | OCH3 | OH | H |
|
* The structures of lichen acids (polyphenolic compounds)
are complex and thus are not shown. They are discussed, for example,
in Huneck and Yoshimura (1996). ** = represents double bond. *** R1 represents CH2C[OH][COOH]CH[OH]COOH where [ ] represents branched group. |
||||||