Cha,H.J., Lee,C.B., Kim,B.S., Choi,M.S.
and Ruttenberg,K.C.(2005): Early diagenetic redistribution
and burial of phosphorus in the sediments of the southwestern
East Sea (Japan Sea). Marine Geology, 216, 127-143.
『東海(日本海)の南西部の堆積物におけるリンの続成作用初期の再分布と埋没』
『Abstract
This study was carried out in order to understand the early diagenetic
redistribution of phosphorus and relevant mass balance in the
sediments of the East Sea. In two cruises during May 1993 and
October 1995, 11 box cores were collected in the southwestern
part of the East Sea. Dissolved phosphorus and iron were analyzed
in the porewater from the cores. Sediment samples were analyzed
for solid-phase P species and solid-phase Fe oxyhydroxide by sequential
extraction.
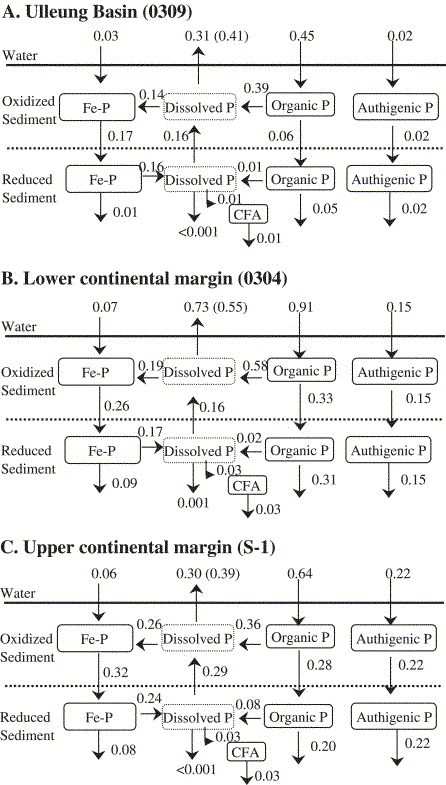
Phosphorus speciation results show that organic P is the major
chemical form of phosphorus in young sediments within the upper
50 cm of sediment. However, the authigenic fraction of total P
increases with depth, indicating the precipitation of carbonate
fluorapatite (CFA) in the sediments. The authigenic CFA (Ca5(PO4)2.6(CO3)0.4F) was formed and buried
at rates of 11-110 μmol cm-2 kyr-1. The
main source of dissolved phosphorus for the precipitation of CFA
is organic P. Dissolved phosphorus, released from the decomposition
of organic P, diffuses upward to return to bottom water, or is
sorbed to iron oxides in the oxidized sediments. As sedimentation
proceeds, the iron oxide-bound P is released in the reduced layer
and enters the dissolved phase, which contributes P to the formation
of CFA in addition to that contributed by the organic P.
The burial flux of reactive P (iron-oxide-bound P + authigenic
P + organic P) is 0.09-0.53 g P m-2 yr-1
that accounts for 18-58% of the reactive P arriving at the sediment/water
interface. The burial flux of reactive P is high in the upper
and lower continental margin sediment. The burial flux of reactive
P in the Ulleung Basin sediment is less than those in the continental
margin sites by a factor of 6, indicating that the reactive P
burial flux is mainly dependent on sedimentation rate.
Keywords: phosphorus; early diagenesis; redistribution; Fe oxides;
carbonate fluorapatite; burial flux; sedimentation rate』
1. Introduction
2. Study area and methods
2.1. Geological setting
2.2. Methods
3. Results
3.1. Total P concentrations
3.2. P concentrations in speciation
3.2.1. Dissolved P in porewater
3.2.2. Detrital apatite-bound P (Det-P)
3.2.3. Iron oxide-bound P (Fe-P)
3.3. Authigenic apatite-bound P (CFA-P)
3.4. Organic P (Org-P)
4. Discussions
4.1. The role of redox cycling of Fe in sedimentary P geochemistry
4.2. Formation of authigenic CFA
4.3. The phosphorus cycle in sediments
5. Conclusions
Acknowledgements
Appendix A. Calculation of fluxes between P reservoirs
References
Table 2 Sequential extraction method for P in sediments
(after Ruttenberg, 1990, 1992)
|
Step |
Extractant |
P component extracted |
|
Adsorbed + iron-bounda |
0.3 M Na-citrate + 1 M NaHCO3 (pH 7.6) 0.5
g Na-dithionite in 20 ml Na-cttrate-bicarbonate solution (8h) |
Exchangeable or loosely aorbed P + easily reducible or reactive
ferric Fe-bound P |
|
Authigenic |
1 M Na-acetate buffered to pH 4 with acetic acid (6 h) |
CFA + biogenic hydroxyapatite + CaCO3-bound
P |
|
Detrital |
1 M HCl (16 h) |
Detritalfluorapatite-bound P |
|
Organic |
Ash at 550℃ 1 M HCl |
Organic P |
|
a This step combines SEDEX steps I and II of Ruttenberg
(1992) (after Rao, 1994). |
ホームへ