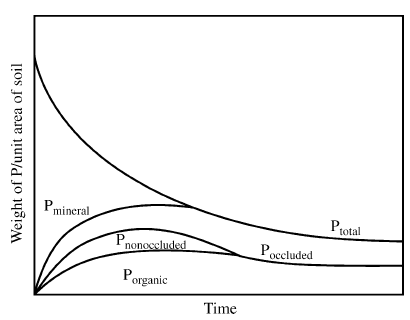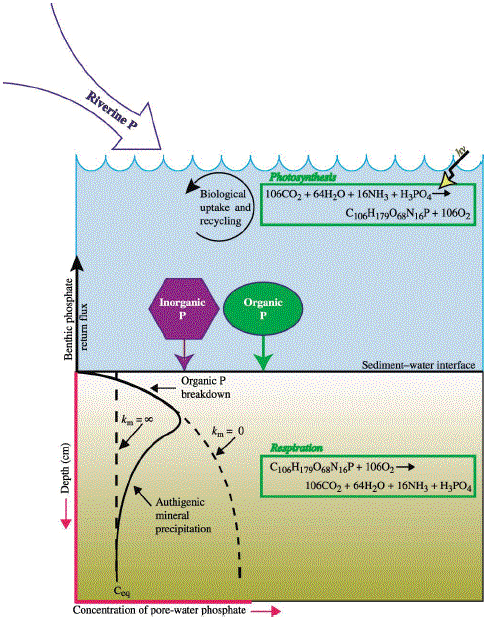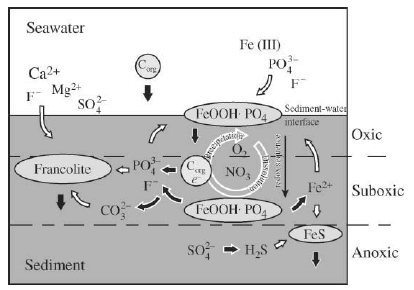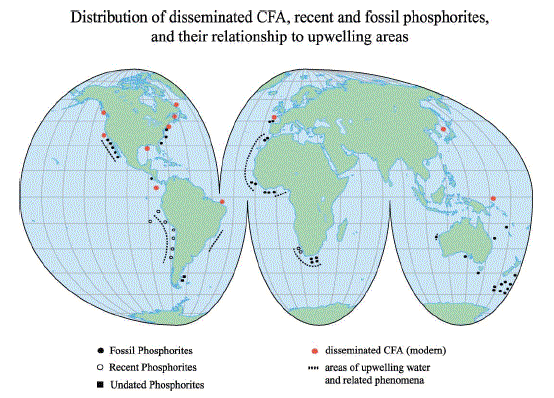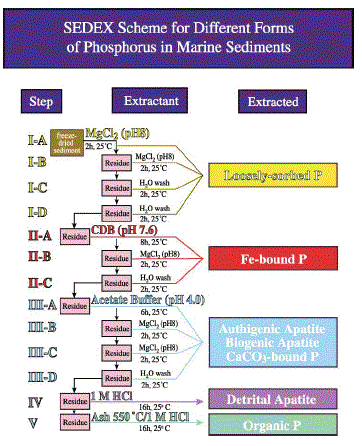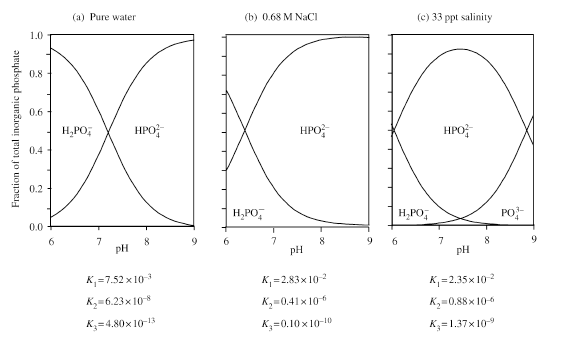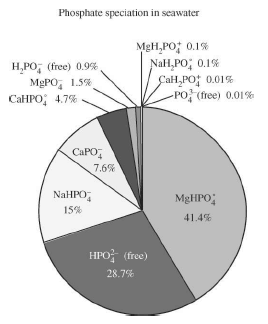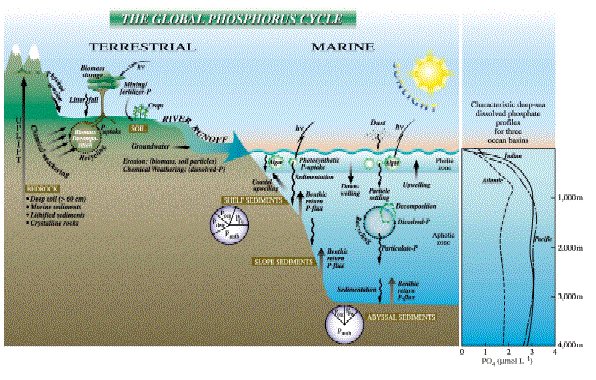
Figure 1 The major reservoirs and fluxes of the global phosphorus cycle are illustrated (see Tables 1 and 2, and text). The oceanic photic zone, idealized in the cartoon, is typically thinner in coastal environments due to turbidity from continental terrigenous input, and deepens as the water column clarifies with distance away from the continental margins. The distribution of phosphorus among different chemical/mineral forms in marine sediments is given in the pie diagrams, where the abbreviations used are: organic phosphorus (Porg), iron-bound phosphorus (PFe), detrital apatite (Pdetr), authigenic/biogenic apatite (Pauth). The Porg, PFe, and Pauth reservoirs represent potentially reactive-P pools (see text and Tables 2 and 3 for discussion), whereas the Pdetr pool reflects mainly detrital apatite weathered off the continents and passively deposited in marine sediments (note that Pdetr is not an important sedimentary phosphorus component in abyssal sediments, far from continents). Continental margin P-speciation data were compiled from Louchouarn et al. (1997), and Ruttenberg and Berner (1993). Abyssal sediment P-speciation data were compiled from Filippelli and Delaney (1996), and Ruttenberg (1990). The “global phosphorus cycle” cartoon is from Ruttenberg (2002). The vertical water column phosphate distributions typically observed in the three ocean basins are shown in the panel to the right of the “global phosphorus cycle” cartoon, and are from Sverdrup et al. (1942).
Ruttenberg,K.C.(2003)による『8.13 The global phosphate cycle』から
図1 世界のリン循環の主なリザーバとフラックスが図示されている(表1と表2と本文を参照)。海洋有光層帯は、図では理想化されているが、大陸の砕屑物の流入による混濁によって沿岸環境では典型的に薄くなっており、大陸縁から離れて水柱が澄むにつれて深くなる。海洋堆積物中の異なる化学的/鉱物学的形態間でのリンの分布は円グラフで与えてあり、使用されている略称は次のとおり:有機リン(Porg)、鉄結合リン(PFe)、砕屑アパタイト(Pdetr)、自生/生物源アパタイト(Pauth)。PorgとPFeとPauthのリザーバは潜在的に反応性Pのプールを示し(議論は本文および表2と表3を参照)、Pdetrのプールは主に大陸から風化で流出した砕屑アパタイトであり、海洋堆積物中に受動的に堆積した(Pdetrは、大陸から離れた遠洋堆積物中の重要な堆積リン成分ではないことに注意)。大陸縁のP化学種データはLouchouarn et al.(1997)およびRuttenberg and Berner(1993)からまとめた。遠洋堆積P化学種データはFilippelli and Delaney(1996)およびRuttenberg(1990)からまとめた。『世界のリン循環』の図はRuttenberg(2002)からのものである。3大海盆で典型的に観測された垂直水柱リン分布は『世界のリン循環』の図の右にパネル画として示されているが、Sverdrup et al.(1942)からのものである。