
Fig. 1. N transformations−emboldened species are the dominant forms for biological assimilation and emboldened processes can be exergonic. Together, ammonification and nitrification often are coined mineralization. Nitrogen species, excluding N2, often are grouped under the heading ‘fixed N’ or ‘reactive N,’ in reference to their common trait of having undergone fixation and being readily available for one or more biological transformations.
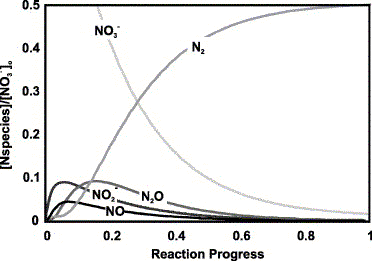
Fig. 7. Ratio of N-species concentration to initial [NO3-] vs reaction progress for the denitrification
reaction sequence of NO3- →
NO2-→ NO → N2O
→ N2 using the Michaelis-Menten half-saturation
constants for Flavobacterium as reported in Betlach and
Tiedje (1981). For much of the reaction period from 〜0.05 to
〜0.1, increasing progress diminishes [NO3-]
at the same time as [N2O] increases and
[N2O-], near its maximum, remains
nearly constant−a qualitatively similar pattern as that observed
for spring SpW2. Note that [N2O] and [N2] are decreased by half relative to other species
to account for reaction stoichiometry. Also note that: (1) the
half-saturation constant for NO, which was not reported by Betlach
and Tiedje (1981), herein is assumed equal to that of N2O; and 2) Betlach and Tiedje (1981) did not
report Michaelis-Menten maximum velocities (v) which vary dramatically
with environmental conditions−to model relative concentrations
similar to our data we used νNO3- = 1, νNO2- = 6, νNO = 1 and νN2O = 0.2.
〔Washington,J.W., Thomas,R.C., Endale,D.M., Schroer,K.L. and Samarkina,L.P.(2006): Groundwater N speciation and redox control of organic N mineralization by O2 reduction to H2O2. Geochimica et Cosmochimica Acta, 70, 3533-3548.から〕