Audry,S., Blanc,G., Schafer(aの頭に¨),J., Chaillou,G. and Robert,S.(2006): Early diagenesis
of trace metals (Cd, Cu, Co, Ni, U, Mo, and V) in the freshwater
reaches of a macrotidal estuary. Geochimica et Cosmochimica
Acta, 70, 2264-2282.
『潮の影響が強い河口の淡水域での微量金属(Cd、Cu、Co、Ni、U、Mo、Vの早期続成作用』
『Abstract
Vertical profiles from the water column, including the maximum
turbidity zone (MTZ) to the consolidated sediment were sampled
in September 2000 in the freshwater reaches of the Gironde Estuary
during a complete neap tide-spring tide cycle. The vertical distributions
of dissolved major redox parameters and metals (Mn, Fe, Cd, Cu,
V, Co, Ni, Mo, and U) were determined. Reactive particulate metal
fractions were also determined from selective leaching. The studied
system is characterized by density layers functioning at different
time-scales, consisting of two mobile layers, i.e., the liquid
(LM) and the soft mud (SM), overlying consolidated sediments (CS).
This results in a three-zone diagenetic regime where (1) O2 dynamics are fast enough to slow depletion in
the rapidly mixed LM sequence (tidal time-scale), (2) denitrification
occurs on the weekly time-scale mixing SM sequence, and (3) the
Mn, Fe, and sulfate cycling occurs in the CS layer (annual time-scale).
The studied trace metals show differential behavior during early
diagenesis: (1) Cd, Cu, and V are released into pore water preferentially
from organic matter in the SM, (2) Co, Ni, and U are released
in the CS from Mn and Fe oxides during reductive dissolution,
and (3) Mo from both processes. Transient conditions (i.e., oscillations
of redox fronts and reoxidation processes), due to the dynamics
of the mobile layers, strongly influence the trace metal distributions
as inducing resolubilization (Cd, Cu, and Mo). In the CS, authigenic
metal phases accumulate, either by direct precipitation with sulfides
(Cu, Cd) or co-precipitation with Fe-sulfides (Mo). Microbially
mediated reduction of Fe oxides is proposed to control U removal
from pore water by reduction of U(VI) to U(IV) at depth. However,
a significant fraction of the trace metals is trapped in the sediment
in exchangeable forms, and therefore is susceptible to be mobilized
due to resuspension of estuarine sediment during strong river
flood periods and/or dredging activities.』
1. Introduction
2. Background and methods
2.1. The Gironde Estuary
2.2. Sample collection and handling
2.3. Pore water analysis
2.4. Solid analysis
2.5. Trace metal measurements
3. Results and discussion
3.1. Water column-consolidated sediment boundary
3.2. Total particulate metals
3.3. Sediment redox conditions
3.3.1. Oxygen and pH
3.3.2.Nitrate
3.3.3. Sulfate and sulfide
3.3.4. Manganese
3.3.5. Iron
3.4. Nonsteady-state diagenesis
3.5. Trace metal behavior during early diagenesis
3.5.1. Cadmium, copper, and vanadium
3.5.2. Cobalt, nickel, uranium, and modybdenum
3.6. Metal sequestration in the Gironde Estuary sediments
4. Conclusion
Acknowledgments
Appendix A. Supplementary data
References

Fig. 2. Schematic representation of the density layers
in the Gironde Estuary water column. MTZ: maximum
turbidity zone; SPM: suspended particulate matter. Abril
et al. (1999).
〔Audry,S., Blanc,G., Schafer(aの頭に¨),J.,
Chaillou,G. and Robert,S.(2006): Early diagenesis of trace
metals (Cd, Cu, Co, Ni, U, Mo, and V) in the freshwater reaches
of a macrotidal estuary. Geochimica et Cosmochimica Acta,
70, 2264-2282.から〕 |
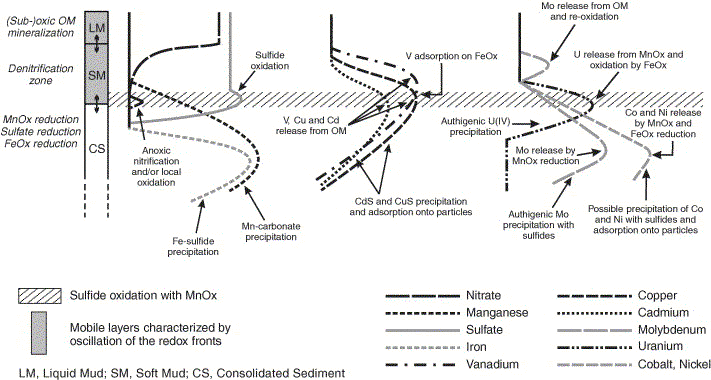
Fig. 11. Schematic conceptual model of the three-zone diagenetic
regime of the sediments of the Gironde Estuary's fluvial reaches
summarizing the transient behaviors of selected trace metals.
The profiles show the evolution of dissolved major redox species
and metals as a function of depth.
〔Audry,S., Blanc,G., Schafer(aの頭に¨),J.,
Chaillou,G. and Robert,S.(2006): Early diagenesis of trace
metals (Cd, Cu, Co, Ni, U, Mo, and V) in the freshwater reaches
of a macrotidal estuary. Geochimica et Cosmochimica Acta,
70, 2264-2282.から〕 |
- Abril,G., Etcheber,H., Le Hir,P., Bassoulet,P., Boutier,B.
and Frankignoulle,M.(1999): Oxic/anoxic oscillations and organic
carbon mineralization in an estuarine maximum turbidity zone
(The Gironde, France). Limnol. Oceanogr., 44,
1304-1315.
- Abril,G., Riou,S.A., Etcheber,H., Frankognoulle,M., de Wit,R.
and Middelburg,J.J.(2000): Transient, tidal time-scale, nitrogen
transformations in an estuarine turbidity maximum-fluid mud system
(The Gironde, South-West France). Estuar. Coast. Shelf
Sci., 50, 703-715.
- Chiffoleau,J.F., Cossa,D., Auger,D. and Truquet,I.(1994):
Trace metal distribution, partition and fluxes in the Seine
estuary (France) in low discharge regime. Mar. Chem.,
47, 145-158.
- Elbaz-Poulichet,F., Garnier,J.M., Guan,D.M., Martin,J.M.
and Thomas,A.J.(1996): The conservative behavior of trace
metals (Cd, Cu, Ni and Pb) and As in the surface plume of stratified
estuaries: example of the Rhone(oの頭に^) river
(France). Estuar. Coast. Shelf Sci., 42, 289-310.
- Guieu,C., Martin,J.M., Tankere,S.P.C., Mousty,F., Trincherini,P.,
Bazot,M. and Dai,M.H.(1998): On trace metal geochemistry in
the Danube river and Western Black sea. Estuar. Coast.
Shelf Sci., 47, 471-485.
- Kraepiel,A.M.L., Chiffoleau,J.F., Martin,J.M. and Morel,F.M.M.(1997):
Geochemistry of trace metals in the Gironde estuary. Geochim.
Cosmochim. Acta, 61, 1421-1436.
- Lasslet,R.E. and Balls,P.W.(1995): The behaviour of dissolved
Mn, Ni and Zn in the Forth, an industrialized, partially mixed
estuary. Mar. Chem., 48, 311-328.
- Morris,A.W., Bale,A.J. and Howland,R.J.M.(1982): The dynamics
of estuarine manganese cycling. Estuar. Coast. Shelf Sci.,
14, 175-192.
- Robert,S., Blanc,G., Schafer(aの頭に¨),J.,
Lavaux,G. and Abril,G.(2004): Metal mobilization in the Gironde
Estuary (France): the role of the soft mud layer in the maximum
turbidity zone. Mar. Chem., 87, 1-13.
- Shink,D.(1981): Behaviour of chemical species during estuarine
mixing. In: Martin,J.M., Burtor,J.D. and Eisma,D.(Eds.),
River Inputs to Ocean System. UNEP/UNESCO, Paris.
- Tamg,D., Warnken,K.W. and Santschi,P.H.(2002): Distribution
and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston
Bay waters. Mar. Chem., 78, 29-45.
- Turner,A. and Millward,G.E.(1993): Partitioning of trace
metals in a macrotidal estuary. Implications for contaminant
transport models. Estuar. Coast. Shelf Sci., 39,
45-58.
- Zwolsman,J.J.G., Van Eck,B.T.M. and Van Der Weijden,C.H.(1997):
Geochemistry of dissolved trace metals (cadmium, copper, zinc)
in the Scheldt estuary, southwestern Netherlands: impact of seasonal
variability. Geochim. Cosmochim. Acta, 61,
1635-1652.
戻る

