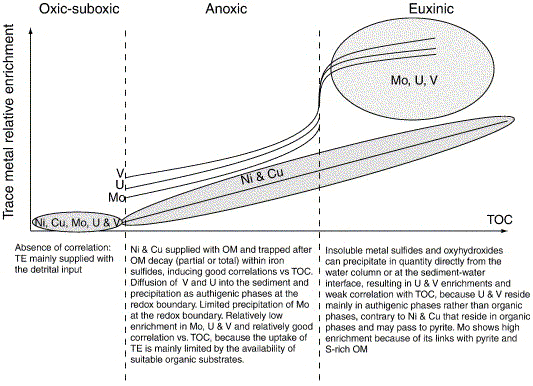
Fig. 2. Schematic diagram illustrating the relative enrichment of Ni, Cu, Mo, U and V versus total organic carbon (TOC). TE stands for trace elements and OM stands for organic matter.
〔Tribovillard,N., Algeo,T.J., Lyons,T. and Riboulleau,A.(2006): Trace metals as paleoredox and paleoproductivity proxies: An update. Chemical Geology, 232, 12-32.から〕
※euxinic=強還元性