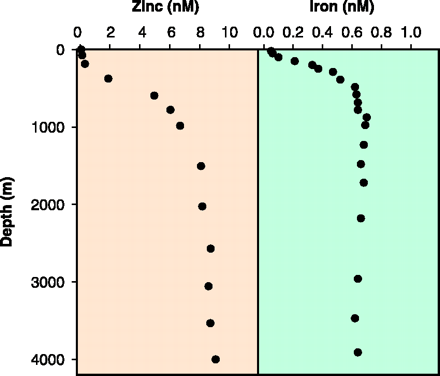
Fig. 1. Vertical profiles of dissolved zinc (43) and iron (44) concentrations in the north Pacific Ocean.
〔Morel,F.M.M. and Price,N.M.(2003): The biogeochemical cycles of trace metals in the oceans. Science, 300, 944-947.から〕
『(Abstract)
Planktonic uptake of some essential metals results in extraordinarily
low concentrations in surface seawater. To sequester or take up
these micronutrients, various microorganisms apparently release
strong complexing agents and catalyze redox reactions that modify
the bioavailability of trace metals and promote their rapid cycling
in the upper water column. In turn, the low availability of some
metals controls the rate of photosynthesis in parts of the oceans
and the transformation and uptake of major nutrients such as nitrogen.
The extremely low concentrations of several essential metals are
both the cause and the result of ultraefficient uptake systems
in the plankton and of widespread replacement of metals by one
another for various biochemical functions.』
(Introduction)
Low surface concentrations of essential metals
Metal chelation
Redox cycle of metals
Limitation by trace metals
References and notes
 Fig. 1. Vertical profiles of dissolved zinc (43) and iron (44) concentrations in the north Pacific Ocean. 〔Morel,F.M.M. and Price,N.M.(2003): The biogeochemical cycles of trace metals in the oceans. Science, 300, 944-947.から〕 |
|
Fig. 2. (A) Examples of release of complexing agents and metal ligand complexes from marine plankton: CdX, phytochelatin-Cd complex released by diatoms (25); CuY, peptide complexes of Cu released by coccolithophorids (26); CuZ, unidentified Cu ligand complex released by Synechococcus (27, 28); sid, siderophore released by heterotrophic bacteria and cyanobacteria (17); L, unidentified Co complexing agent released by Prochlorococcus (29); C, Cys; E, Glu; G, Gly; Q, Gln; and R, Arg. (B) Redox cycling of Fe and Mn via photochemical and biochemical processes. Diatoms extracellularly reduce Fe(III) ligand complexes during Fe uptake (33, 34); heterotrophic marine bacteria oxidize Mn(II), forming a MnO2 casing around the cell (38, 39). 〔Morel,F.M.M. and Price,N.M.(2003): The biogeochemical cycles of trace metals in the oceans. Science, 300, 944-947.から〕 |
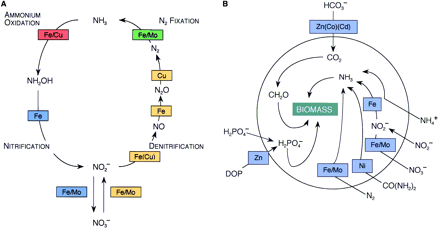 Fig. 3. (A) A diagram of the nitrogen cycle, illustrating the metal cofactors in each enzymatically catalyzed step. Color coding identifies the sets of reactions involved in nitrogen fixation (green), denitrification (gold), nitrification (blue), and ammonium oxidation (red). All the metals shown here (with the exception of Mo, which has a concentration of 0.1 μM) are depleted in surface seawater. (B) Primary metal requirements for carbon, nitrogen, and phosphorus acquisition and assimilation by marine phytoplankton. 〔Morel,F.M.M. and Price,N.M.(2003): The biogeochemical cycles of trace metals in the oceans. Science, 300, 944-947.から〕 |