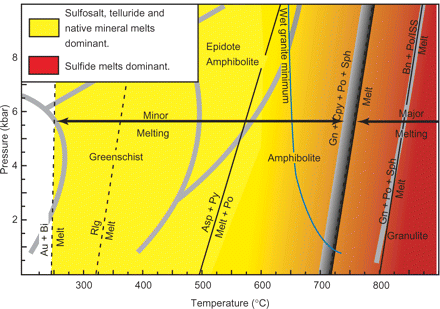
Fig. 3. The temperature-pressure range over which sulfosalts, tellurides, native minerals and sulfides melt. Continuous black lines indicate that pressure constraints are known; dashed black lines indicate that the effect of pressure is unknown. Wide grey lines separate the metamorphic facies [the amphibolite to granulite transition is from Pattison et al. (2003) and the other facies boundaries are from Spear (1993)]. The wide band separating minor from major melting represents the variation caused by differences in the natural environment in the amount of H2O present (Wykes & Mavrogenes, 2005), and in the amount of trace metals present, on an assemblage containing galena+chalcopyrite+pyrrhotite+sphalerite. There will be widespread melting of the major sulfides at lower temperatures in wet, trace metal-rich rocks. The melting curve of galena+troilite (stoichiometric FeS)+sphalerite plots in the same position as that for bornite+ISS and bornite+pyrrhotite (Fe1-xS).
〔Tomkins,A.G., Pattison,D.R.M. and Frost,B.R.(2007): On the initiation of metamorphic sulfide anatexis. Journal of Petrology, 48(3), 511-535.から〕