Abrecht,J.(1985): Manganiferous pyroxenes
and pyroxenoids from three Pb-Zn-Cu skarn deposits. Contrib.Mineral.Petrol.,
89, 379-393.
Abstract
Introduction
Geologic setting of the deposits
Empire Mine (and Princess Mine)
Monte Civillina
Valle del Temperino
Description of samples
Empire Mine (and Princess Mine)
Microscopic description
Clinopyroxene
Bustamite
Rhodonite
Andradite
Monte Civillina
Microscopic description
Clinopyroxene:
Rhodonite
Valle del Temperino
Microscopic description
Clinopyroxene
Bustamite
Other phases
Clinopyroxene-rhodonite assemblages
Mineral chemistry
Clinopyroxenes
Bustamite
Rhodonite
Andradite
Genetic considerations
Acknowledgments
References
Abstract
Samples from the Pb-Zn-Cu skarns of M. Civillina (Italy), Valle
del Temperino (Italy), and Empire Mine (New Mexico, USA) have
been analysed for their pyroxenes and pyroxenoids. The samples
were collected immediately adjacent to the marble-skarn replacement
front. All contain manganiferous pyroxenoids and manganeserich
Ca-pyroxenes. The pyroxenes from each deposit form distinct groups
of compositions within the diopside-hedenbergite-johannsenite
triangle, with no apparent miscibility gap. Diopside contents
usually are below 15 mole percent. Fibrous bustamite occurs as
monomineralic zones in the Empire and in the Temperino deposit.
Although rhodonite may be a primary phase in some samples from
the Empire Mine, it is commonly of secondary origin in the Empire
Mine and in the Civillina deposit. Its formation from manganiferous
clinopyroxenes is either due to increasing Mn activity in the
hydrothermal skarn solution or to higher X(CO2)
in the vapour phase. When rhodonite is formed within clinopyroxenes
as submicroscopic lamellae that eventually replace the whole host
crystal, resulting compositions lie in the miscibility gap between
rhodonite and bustamite. Textural relations indicate the replacement
reaction: johannsenite + CO2 = rhodonite
+ calcite + quartz. Equilibrium temperatures for this reaction
have been calculated by using estimated thermochemical data for
johannsenite, giving a T(eq)= 385℃ for X(CO2)=
0.1 at P(tot)= 1 kbar. Taking into consideration the reduced activity
of Mn in rhodonite and of Ca in calcite, both buffered by
the johannsenite, the temperature is increased for about 15℃ at
X(CO2)= 0.01. At lower temperatures, where
johannsenite is stable, the X(CO2) is confined
to values below 0.01. Despite the mineralogical similarities of
the three deposits differences in the development of the manganiferous
skarns can be depicted.

Fig. 8. T−XCa diagram of the system CaO-MnO-SiO2-CO2 for low X(CO2) and low pressures. Constructed from data by
Maresch and Mottana (1976), Abrecht and Peters (1980), and Peters
et al. (1980)
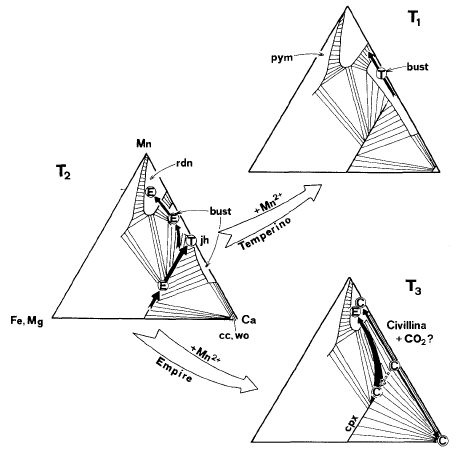
Fig. 11. Possible phase relations in isobaric-isothermal sections
in the
system CaO - MnO - (Fe,Mg)O - SiO2-CO2 constructed from data by Abrecht (1980) and
Brown et al. (1980). Circles represent compositions of samples
from Empire Mine (E), Valle del Temperino (T), and M. Civillina
(C). Black arrows indicate compositional trends suggested by
analytical data and reactions inferred from textures. The white
arrows refer to possible paths of skarn development at the temperatures
T1, T2, and T3 taken from Fig. 8. These temperatures are only
relative and demonstrate development within single deposits
Abrecht.(1985)による『Manganiferous
pyroxenes and pyroxenoids from three Pb-Zn-Cu skarn deposits』から |
戻る

