Maresch,W.V. and Mottana,A.(1976): The
pyroxmangite-rhodonite transformation for the MnSiO3 composition. Contrib.Mineral.Petrol.,
55, 69-79.
Abstract
1. Introduction
2. Experimental methods and starting materials
3. Characterization of phases encountered
4. Previous experimental work
5. Present experimental results
6. Petrological application
Acknowledgements
References
Abstract
The polymorphic transformation between synthetic pyroxmangite
and rhodonite of MnSiO3 composition has been
reversibly bracketed in the presence of water at 3 kbar (between
425゜ and 450 ℃), 6 kbar (betwee 475゜and 525℃), 20 kbar (between
500゜and 900℃), 25 kbar (between 800 ゜and 900℃) and 30 kbar (between
900゜and 1,000℃), using standard cold-seal pressure vessels and
piston cylinder apparatus. Oxygen fugacities buffered by the bomb
walls and piston-cylinder cell assemblies sufficed to keep manganese
in the divalent state. Pyroxmangite of MnSiO3
composition is shown to be the high-pressure, low-temperature
polymorph with respect to rhodonite of the same composition. It
is a stable phase at atmospheric pressure below 350-405℃.
X-ray data for synthetic pyroxmangite are presented. The unit-cell
parameters (ao =6.717(2)Å, bo
= 7.603(1)Å, co = 17.448(5)Å, α= 113゜50'(1'),
β=82゜21'(2'), γ=94゜43'(1'); space group P1(1の頭に-))
give a unit-cell volume (807.5(0.3)Å3) which, in accordance
with other recent least squares lattice refinements of hydrothermally
synthesized material, is slightly smaller than that obtained by
single-crystal work on anhydrously synthesized material.
Application of the present results to natural rocks is severely
restricted due to the great variety and extent of cationic substitutions
observed in natural pyroxenoids. The univariant polymorphic transformation
determined for the MnSiO3 composition is
thus replaced in natural systems by a divariant field in which
pyroxmangite and rhodonite of differing composition will stably
coexist.
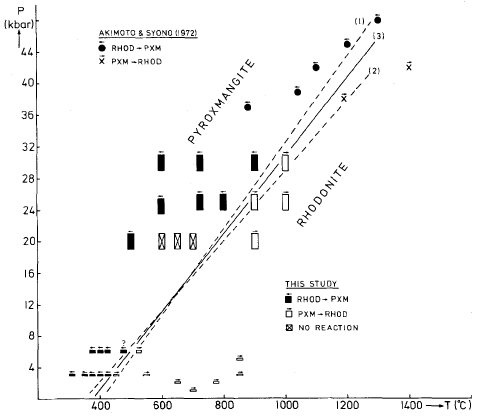
Fig. 1. Experimental results of this study. Size of symbol for
our runs reflects experimental uncertainties in temperature and
pressure. For equations of curves (1), (2), and (3) see text.
Because the position of the solidus in the hydrous system is
not known, it is possible that the high-pressure part of the
transformation curve defined by the data of Akimoto and Syono
(1972) may be metastable in the presence of water
Maresch & Mottana(1976)による『The
pyroxmangite-rhodonite transformation for the MnSiO3
composition』から |
戻る
