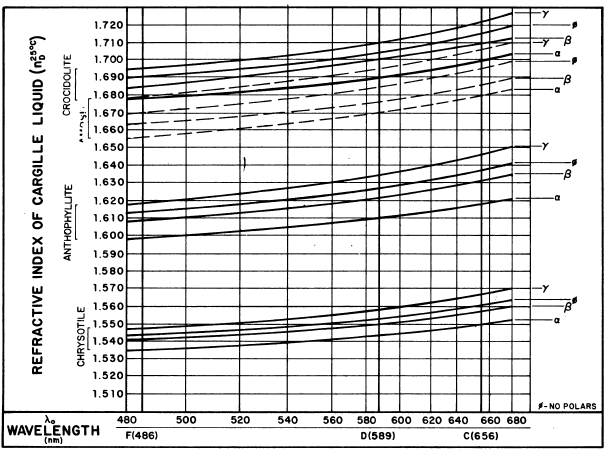
FIGURE 1. Asbestos dispersion staining curves.
McCrone(1974)による『Detection and identification of asbestos by microscopical dispersion staining』から
Asbestos fibers as small as 1 ,m in diameter can be uniquely identified by light microscopy by employing dispersion staining methods. The technique described herein involves suspension of fibers in liquids ofknown refractive indices and observation of color display by means of a dispersion staining objective. Wavelengths or indices of refraction may be determined at right angles to and parallel to fiber axes. This method is rapid and sensitive for identification purposes.
There is a great need for a dependable, sensitive and rapid
method for the detection and identification of asbestos. Microscopical
dispersion
staining satisfies all of these requirements. It is dependable
because it is based on the measurement of three refractive indices
as well as the dispersion of those indices. Refractive indices
are among the most valuable identifying characteristics for small
particles. The method is sensitive because the refractive indices
are "read" as bright dispersion staining colors against
a black background. These colors can be observed on particles
well below 1 Am in diameter. It is rapid because any particle
in the microscopical preparation showing the optical properties
of asbestos signals its presence by a unique color combination
with polarized light. One particle of asbestos in a field of view
containing many thousands of other particles will be immediately
apparent on scanning the eye across the field of view.
Besides optical crystallographic methods like dispersion staining,
only differential thermal analysis (DTA) and X-ray diffraction
have this ability to tag a particular crystalline phase in a mixture.
X-ray diffraction, however, is several orders of magnitude less
sensitive than dispersion staining and requires much more time.
There are, however, compounds whose dispersion staining colors
are, at least at first glance, confused with chrysotile colors.
Here, fortunately, particle morphology is able to differentiate
between these interfering substances. Quartz is one example, lizardite
another. The latter, however, is a tabular talclike mineral, and
quartz shows conchoidal fracture into usually thin flakes. Both
are easily differentiated from fibrous asbestos by morphology.
Dispersion staining is, therefore, a straightforward technique
easily applied by any microscopist with some knowledge of optical
crystallography. We have found it extremely useful for the rapid
and routine examination of any particulate samples for any of
the various kinds of asbestos (1). The necessary background information
for applying the method is given in Figure 1, which plots the
matching wavelength Xo, as a function of refractive index of the
Cargille refractive index liquids used in these determinations.
Most of the asbestos minerals have distinctive indices without
overlap. Figure 1 suggests, however, that amosite and crocidolite
may overlap partially. There should be no confusion in this situation,
however, since y for amosite overlaps only with a for crocidolite.
The data in Figure 1 are plotted as the averages of a considerable
number of values December 1974 for individual mine samples previously
published (1). It is interesting to look a little bit more closely
at this variation in dispersion staining data from mine to mine,
and Table 1 lists the matching wavelength λo for γ (parallel to
the fiber length) and α (perpendicular to the fiber length) for
a group of more than 30 asbestos samples from different parts
of the world. There is some variation from sample to sample, indicating
composition variations. However, all of the data lie in the same
characteristic chrysotile region and show no overlap with any
of the fibrous amphiboles.
Since dispersion staining is a relatively new technique requiring
a certain amount of skill, not only in reading the matching wavelengths
but also in adjusting the microscope for best dispersion staining
colors, it seems well to summarize a few of the common difficulties.
The refractive indices given in dispersion staining tables are
not dispersion data for that compound. To illustrate this, we
can cite the data for the ω index of quartz (Table 2).
The value 1.538 for ω at 486 nm is nD for
the Cargille liquid that matches quartz ω at 486 nm. The actual
refractive index of that liquid at 486 nm is 1.550, the same as
quartz ω). This operation, which simplifies the analytical procedure,
is used for all dispersion staining data. One can, of course,
calculate the true refractive indices of any substance from the
dispersion staining data. The necessary data to do this can be
found in the table of dispersion of refractive index data for
the Cargille liquids.
True refractive index data and dispersion staining data are identical
at 589 nm; hence, refractive index data at 589 nm for any substance
become dispersion staining data for that substance. Chrysoberyl,
for example, does not appear in the dispersion staining tables,
but Winchell (2) gives nD = 1.746 (α), 1.748
(β), and 1.756 (γ). From these data one would mount a suspected
chrysoberyl in Cargille liquid nD = 1.750
and expect to see annular stop colors ranging from orange (β)
to greenish-yellow (γ) or greenish-blue (α) to magenta-blue (γ)
with the central stop. This greatly extends the usefulne of dispersion
staining.
We are often asked if the dispersion staining objective can be
supplied with a higher magnification. This gives us the opportunity
to point out that higher magnification is not desirable. One is
trying to "resolve" color of the particles not the particles
themselves. Central stop dispersion staining is a darkfield procedure,
hence a strong light source will show points of light for particles
smaller than the resolving power limit of the microscope system,
i.e., 1.22 μm for NA= 0.25, 10× objective. These points of light
will be colored for particles showing dispersion in that liquid.
The lower limit of detection is a function of light intensity
and contrast.
There are a number of points of technique which greatly improve
the sensitivity of the dispersion staining procedure. The particles
must be well separated in the mounting liquid since nonstained
particles close to or overlapping stained asbestos can mask their
presence. The dispersion staining colors for chrysotile and the
fiber amphiboles are more brilliant if one uses the high dispersion
Cargille set of refractive index liquids. In spite of the above
injunction concerning high magnification, it is sometimes useful
to use a 20-25× ocular with the 10× dispersion staining objective.
The optics for the dispersion staining objective, the axis of
stage rotation, the substage apertures and lenses must be well
aligned on the same optical axis. It is a good idea to take special
pains to align the optical system and to maintain that microscope
for dispersion staining examination only. The problem of glare
from other particles in the field of view is solved to a great
extent by having a centered and nearly closed field diaphragm
in the optical system. This concentrates attention on particles
over 90% of the glare which makes it difficult to see very fine
asbestos fibers. It is desirable to be able to change the orientation
of any particles which appear at first sight to give the distinctive
dispersion staining colors characteristic of chrysotile (or other
fibrous amphiboles). Rolling the particle by sliding the cover
slip with a viscous liquid prep is the ideal way of doing this
and helps greatly in differentiating quartz, paper fibers and
mineral wool from chrysotile. The slides and cover slips used
for dispersion staining preparations should be unusually clean
since any optical discontinuities on any surface of the preparation
cause glare and interfere with visibility of the dispersion staining
colors. Finally, it is desirable to have standards of the substance
you are looking for mounted in the same refractive index liquid.
If one is looking for chrysotile, it is also useful to have standard
preparations of quartz, paper fibers, and talc in
order to remind oneself quickly of the essential differences in
appearance and color for these substances.
The color plates (Figs. 2-16) show the general nature of the dispersion
staining colors and the specific appearance of the various kinds
of
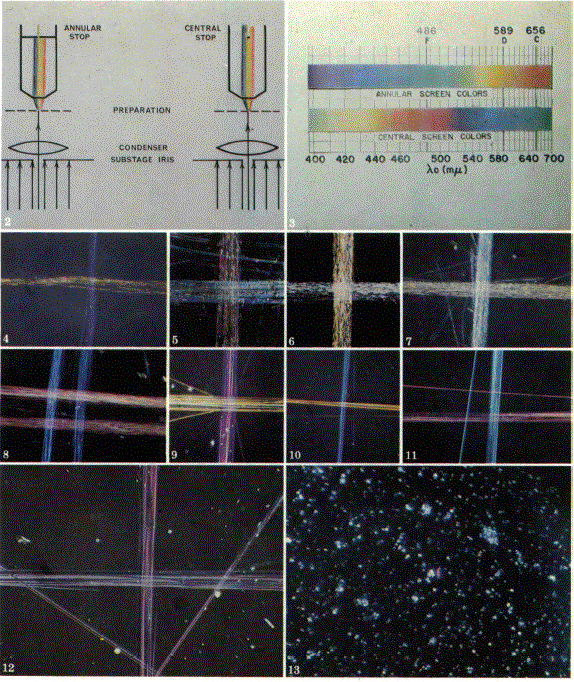
asbestos in their specific liquids. Figure 2 shows the arrangement
of annular and central stops in the objective back focal plane.
These may be a
centered 2-3 mm opening in any opaque film or a 3-4 mm dot of
India ink on an 18 mm cover slip, respectively; although a dispersion
staining
objective is available commercially. Note that the substage iris
is closed to allow only an axial beam of light to strike the object.
The color series obtained with each stop are shown in Figure 3.
Chrysotile is shown in Figures 4 and 5 mounted in two different
standard Cargille refractive index media. Polarized light is used
with an E-W vibration direction for Figure 4 and N-S for Figure
5. Observed Xo values are given in Table 3.
Figures 6-8 show anthophyllite all with E-W polar. Figures 6 and
7 show the fibers mounted in 1.610 and 1.620 refractive index
media, respectively. Figure 8 shows two different anthophyllites
(from Maryland and North Carolina) mounted in liquid of nD 1.627. This figure, like the others, is a double
exposure with the stage rotated 900 between exposures. The two
different sources result in small variations in λo.
Amosite is shown in Figures 9-11 mounted in Cargille liquids of
nD1.670, 1.680, and 1.690, respectively. Figure 12 shows crocidolite
in Cargille
liquid nD = 1.700, and Figure 3 shows one
particle of chrysotile in a talc sample mounted in Cargille liquid,
nD = 1.555.
Conclusion
The combination of particle morphology and optics uniquely identifies
any of the fibrous asbestos compounds. The most rapid method for
obtaining this information is through the use of dispersion staining.
Properly carried out, the dispersion staining method is capable
of sensitivity in the ppm range.
REFERENCES
 FIGURE 1. Asbestos dispersion staining curves. McCrone(1974)による『Detection and identification of asbestos by microscopical dispersion staining』から |
 2. Schematic arrangement for annular and central
stop dispersion staining McCrone(1974)による『Detection and identification of asbestos by microscopical dispersion staining』から |