Golubev et al.(2006)による〔『Effect of pH and
organic ligands on the kinetics of smectite dissolution at 25℃』(4436p)から〕
『25℃でのスメクタイト溶解のカイネティックスに対するpHと有機配位子の影響』
『Abstract
Forward dissolution rates of Na-Montmorillonite (Wyoming) SWy-2
smectite (Ca0.06Na0.56)[Al3.08Fe(III)0.38Mg0.54]
[Si7.93Al0.07]O20(OH)4 were measured at
25℃ in a mixed-flow reactor equipped with interior dialysis compartment
(6-8 kDa membrane) as a function of pH (1-12), dissolved carbonate
(0.5-10 mM), phosphate (10-5 to 0.03 M), and nine organic
ligands (acetate, oxalate, citrate, EDTA, alginate, glucuronic
acid, 3,4-dihydroxybenzoic acid, gluconate, and glucosamine) in
the concentration range from 10-5 to 0.03 M. In organic-free
solutions, the Si-based rates decrease with increasing pH at 1≦pH≦8
with a slope close to -0.2. At 9≦pH≦12, the Si-based rates increase
with a slope of 〜0.3. In contrast, non-stoichiometric Mg release
weakly depends on pH at 1≦pH≦12 and decreases with increasing
pH. The empirical expression describing Si-release rates [R,
mol/cm2/s] obtained in the present study at 25℃, I
=0.01 M is given by
R = 2.2・10-17・aH+0.21 + 1.0・10-20 + 6・10-17・aOH-0.33
At circum-neutral pH, the Si-release-based dissolution is promoted
by the addition of the following ligands ranked by decreasing
effectiveness: EDTA>3,4-DHBA>citrate≧oxalate. Phosphate, glucuronate,
glucosamine, gluconate, alginate, and acetate act as inhibitors
of dissolution and HCO3-, CO32- exhibit no effect on dissolution
rate. Non-stoichiometric, non-steady-state Mg release was very
weakly affected by the presence of ligands. Analysis of reacted
solid products using XRD, FT-IR, and XPS revealed no major change
in structure, surface chemical composition or specific surface
area as a function of pH, ligand concentration, and duration of
experiments. Ligand-affected rates re-calculated to constant pH
were interpreted using a phenomenological equation which postulates
the Langmurian adsorption of a ligand on surface sites. Overall,
results of this study demonstrate that very high concentrations
(0.001-0.01 M) of organic ligands, whether they are originated
from organic matter enzymatic degradation or bacterial metabolic
activity are necessary to appreciably affect smectite dissolution.
As a result, the effect of natural organics on the weathering
rate of smectite is expected to be weak.』
1. Introduction
2. Materials and methods
2.1. Starting clay
2.2. Dissolution kinetics experiments
2.3. Solution analysis
2.4. Solid phase preparation for the analyses
2.5. X-ray photoelectron spectroscopy (XPS)
2.6. Microwave digestion
2.7. Infra-red spectroscopy
2.8. X-ray diffraction
2.9. Scanning electron microscopy
2.10. Specific surface area measurements
3. Results and discussion
3.1. Solid phase characterization
3.2. Steady-state attainment and the stoichiometry of dissolution
reaction
3.3. Effect of pH on smectite dissolution rates
3.4. Effect of ligands on smectite dissolution rates
3.5. Implications for smectite weathering in natural environment
4. Concluding remarks
Acknowledgments
Appendix A
References
|
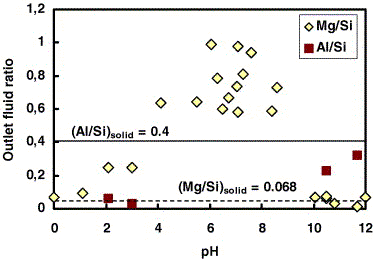
Fig. 3. Molar Mg/Si (diamonds) and Al:Si (squares) ratio
in the outlet fluid after 1000-1500 h of reaction as a function
of pH. Stochiometric dissolution is observed only in strongly
acidic and alkaline solutions. Adsorption of Al on the interlayer
positions at pH < 4.5 and proton-octahedral magnesium exchange
at 2 < pH < 10 are suggested to explain this non-stoichiometry
(see Section 3.2).
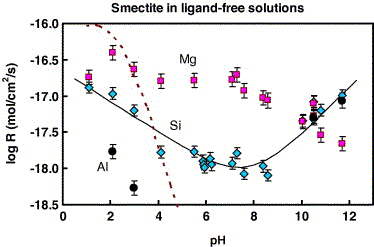
Fig. 4. Summary of smectite dissolution at 25℃ as a function
of pH in CO2-free and CO2-bearing
solutions. Diamonds, Si-based rates; squares, non-stoichiometric
Mg release; circles, Al-based rates. The solid line represents
the fit of experimental data to Eq. (3) (see explanation in the
text). Thick dash line corresponds to Amram and Ganor (2005)
data.
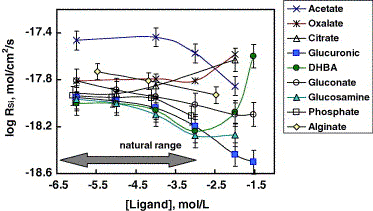
Fig. 5. Silica-release-based dissolution rates of smectite
SWy-2 in organic-bearing solutions at 25℃, I = 0.01 M
and constant pH for each ligand (average pH = 6.08 ± 0.45, see
Table 1 and Table 2). The symbols represent the experimental
data and the solid lines are for guiding purposes. For acetate
and oxalate it is postulated that ligand-free experiments rates
are equivalent to 10-6 M oxalate or acetate since
no effect is observed till 10-4 M.
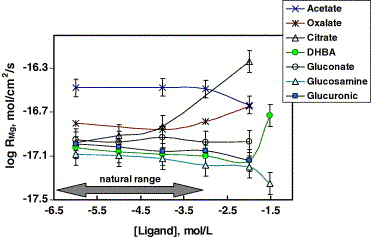
Fig. 6. Rates of non-stoichiometric magnesium release from
SWy-2 smectite in organic-bearing solutions at 25 ℃, I
= 0.01 M and pH = 6.08 ± 0.45. The symbols represent the
experimental data and the solid lines are for guiding purposes.
〔Golubev,S.V., Bauer,A. and Pokrovsky,O.S.(2006): Effect
of pH and organic ligands on the kinetics of smectite dissolution
at 25℃. Geochimica et Cosmochimica Acta, 70,
4436-4451.から〕 |
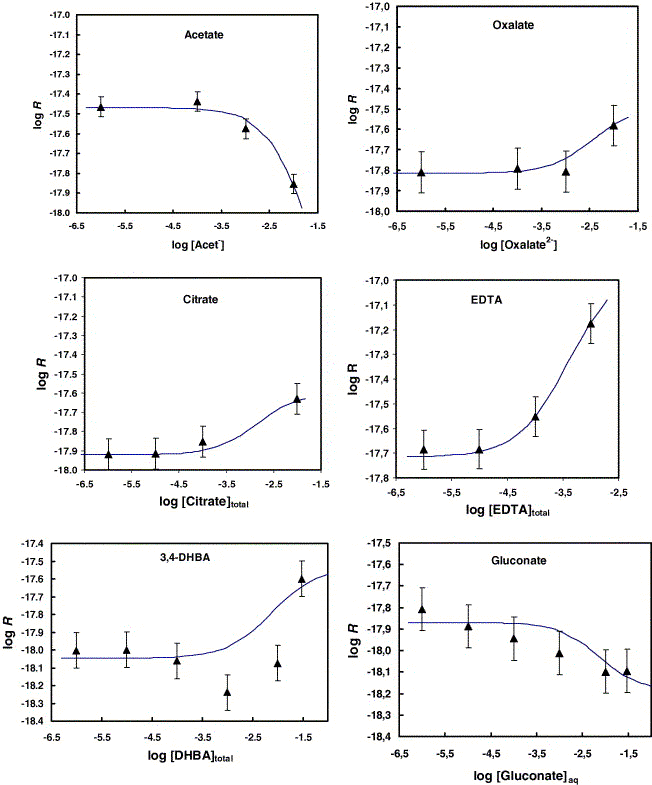
Fig. 7. Effect of added ligands on smectite Si-based steady-state
dissolution rates. Triangles represent Si-release-based rates
and the solid lines represent the fit to the data using Eq. (8a)
and (8b) with parameters listed in Table 2.
〔Golubev,S.V., Bauer,A. and Pokrovsky,O.S.(2006): Effect
of pH and organic ligands on the kinetics of smectite dissolution
at 25℃. Geochimica et Cosmochimica Acta, 70,
4436-4451.から〕 |
戻る




