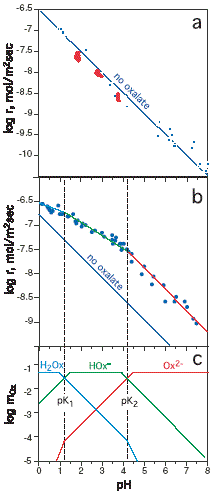
FIGURE 1. Rates of forsterite dissolution.
(a) reported dissolution rates as a function of pH in the
absence of organic ligands (Pokrovsky and Schott 2000b; Rosso
and Rimstidt 2000).
(b) comparison of our measured rates in 〜0.02 M oxalate
solutions with the rates in the absence of organic ligands.
(c) speciation of oxalate as a function of pH shows that
the pH dependence of the rates decreases as oxalate anions hydrolyze
to bioxalate and oxalic acid suggesting a much weaker effect
of bioxalate anion on rates.
〔Liu et al.(2006)による〔『Mechanism for the dissolution of olivine series minerals in acidic solutions』(455p)から〕